Surface Tension Is Same for Different Liquids Explain
Benzene C 6 H 6. The surface tensions of a few common liquids and solutions are as follows in dynecm note the particularly high surface tension of water.
Surface Tension Of Water Why Is It So High
Surface tension is not only determined by the forces of attraction between particles but also by the forces of attraction of solids liquids and gases in close proximity to it.

. Shape of liquid meniscus Due to surface tension the. What that means is if you were to draw an imaginary line on the surface of a liquid film or bubble and then attempt to pull it apart the force required to pull and split the liquid at that line divided by the length of the line would be the. Glycerin C 3 H 2 OH 3.
Surface tension not only depends upon the forces of attraction between the particles within the given liquid but also on the forces of attraction of solid liquid or gas in. Other examples of surface tension include water striders dew drops raindrops on a. Surface tension is literally the force required to overcome these attractions and pull apart molecules that are present on the surface of a liquid.
Dispersive and polar hydrogen bonding acid-base contributions etc corresponding single references and data at elevated temperatures up to 180 C and surfactant property prediction CMC surface tensionCMC please send an email to the address below. It could be liquid-liquid liquid-solid or solid-air. Surface tension is caused by attraction between liquid molecules.
Surface tension is the amount of energy required to increase the surface of the liquid by unit area. Surface tension is measured as the force acting normally per unit length on an imaginary line drawn on the free liquid surface at rest. Surface energy surface tension x change in surface area.
It is represented by the symbol T or S. It combines the concepts of cohesion and adhesion. Partly due to the strength of the force that bids the molecules together.
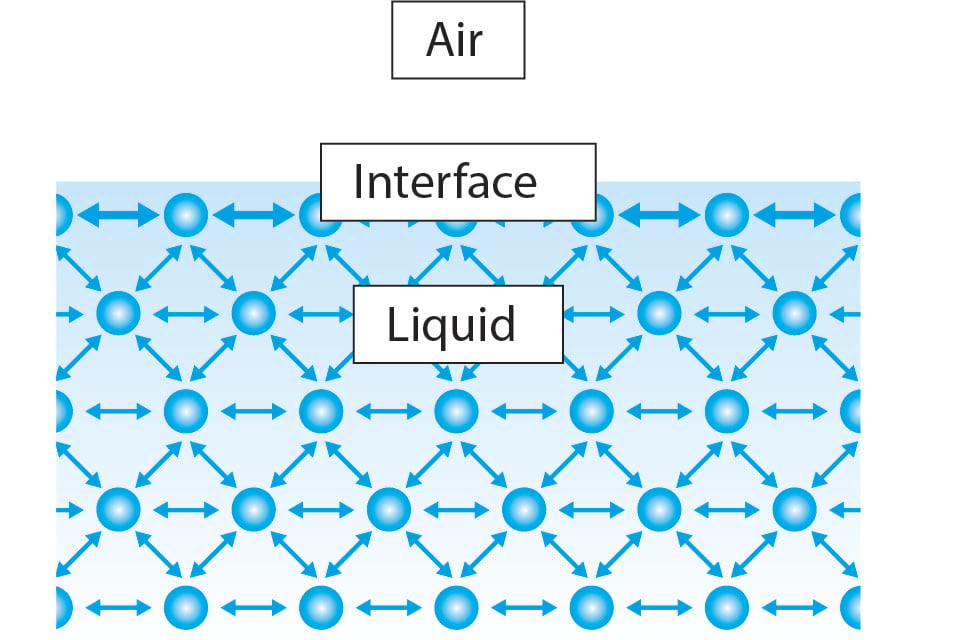
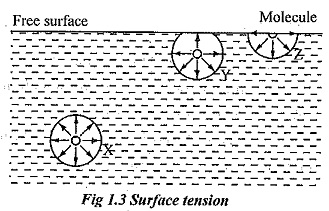
A microscopic view of water illustrates the difference between. Intuitively it keeps a barrier between foreign materials and liquid as well as this is the force that holds the liquid molecules bind together. A molecule lying inside the liquid is surrounded by other molecules and.
Many liquids have what is called hydrogen bonding and water has a high value for hydrogen bonding. Surface tension is an important factor in the phenomenon of capillarity. The best example of liquid surface tension may be witnessed when a small ant becomes entangled in a drop of water even with it being in just a drop of water.
The ant has to struggle to free itself from the liquid due to the force of surface. Interfacial tension on the other hand is the property between any two substances. Find Surface tension of different liquids at different temperature like surface tension of Acetic acid Acetone Diethyl ether Ethanol Glycerol n-Hexane Hydrochloric acid Isopropanol Liquid Nitrogen Mercury Methanol n-Octane Sucrose Water and Toluene at different temperature.
Because of the relatively high attraction of water molecules to each other through a web of hydrogen bonds water has a higher surface tension 728 millinewtons mN per meter at 20 C than most other liquids. Why bubbles are round. Although easily deformed droplets of water tend to be pulled.
The tendency to minimize that wall tension pulls the bubbles into spherical shapes. With surface tension being a line force it is normally expressed as force per unit length drawn across the free liquid surface. Surface tension is caused by a strong attraction between the molecules cohesion that cause them to link together and remain uniform even when placed on differing surfaces adhesion.
Surface tension and droplets. Surface tension is responsible for the shape of liquid droplets. Surface tension is the tension of the surface film of a liquid caused by the attraction of the particles in the surface layer by the bulk of the liquid which tends to minimise surface area.
Unit is newton per metre N m-1. The surface tension of water provides the necessary wall tension for the formation of bubbles with water. When the molecules possess.
The surface tension of a liquid results from an imbalance of intermolecular attractive forces the cohesive forces between molecules. The same considerations apply to liquid-liquid interfaces. Surface tension is a phenomenon in which the surface of a liquid where the liquid is in contact with a gas acts as a thin elastic sheet.
This term is typically used only when the liquid surface is in contact with gas such as the air. However note that the equality of interfacial energy and interfacial tension only applies to liquids because the atomsmolecules in liquid phases can be displaced without performing additional work. The surface tension keeps the surface intact so the whole liquid surface is dragged upward.
Units of Surface Tension. See diagram on the right. A molecule at the surface of a liquid experiences only net inward cohesive forces.
Thus water and ethanol have strong surface tension. Thus for liquids the surface tension and the surface energy are equal. Surface tension is the property of the liquid in contact with gas phase usually air.
This liquid feature is based on the fact that the molecules of the liquid at the surface area are in a different state than those in the center of the liquid. Different compounds have different levels of attraction. Different liquids and solutions have different surface tensions.
In other words it is also the property of the liquid surface that resists force. Here the water molecules in the droplet are held together by surface tension while they are in contact with the desk. Surface tension is the property of liquid which arises due to the fact that the molecules of the liquid at the surface are in a different situation than those in the interior of the liquid.
No liquids have different surface tensions. Then the weight of the risen liquid is balanced by the surface tension. The maximum height to which the liquid will rise through capillary action is given by.
Surface Tension of Different Liquids By. This relation between the polarity of a molecule and surface tension means that liquids of more polar molecules can have larger surface tensions. - For surface tension components eg.
This can be seen in ethanol C 2 H 5 OH which has a weaker surface tension than water because the C 2 H 5 group in ethanol is non-polar and the polarity of the OH group is weaker than the polarity of water. Surface and interfacial tension are usually presented by the symbol σ and it is measured by force per unit length. Its dimensional formula is M 1 LT-2.
A molecule in the bulk liquid experiences cohesive forces with other molecules in all directions. VanDerWahls forces are anothe force that determins surface tension the stronger the more the surface tension. Aliya Abbas Table Changes More substances added to water Having a bigger area to test the surface tension Results Post-Research Hypothesis Summary If salt is addded to water then water molecules will loosen or break because salt is.
If the surface is between two liquids such as water and oil it is called interface tension. Sucrose solution 85 in water. Surface tension is the force that holds atoms or molecules of the same substance together when they are in contact with another substance.
Surface tension is a property of a liquid that allows them to resist external forces. Water and Ethanol are held together by hydrogen bonds the strongest of all intermolecular forces.
Surface Tension Surface Tension Of Liquids Surface Tension In Soap Bubble
No comments for "Surface Tension Is Same for Different Liquids Explain"
Post a Comment